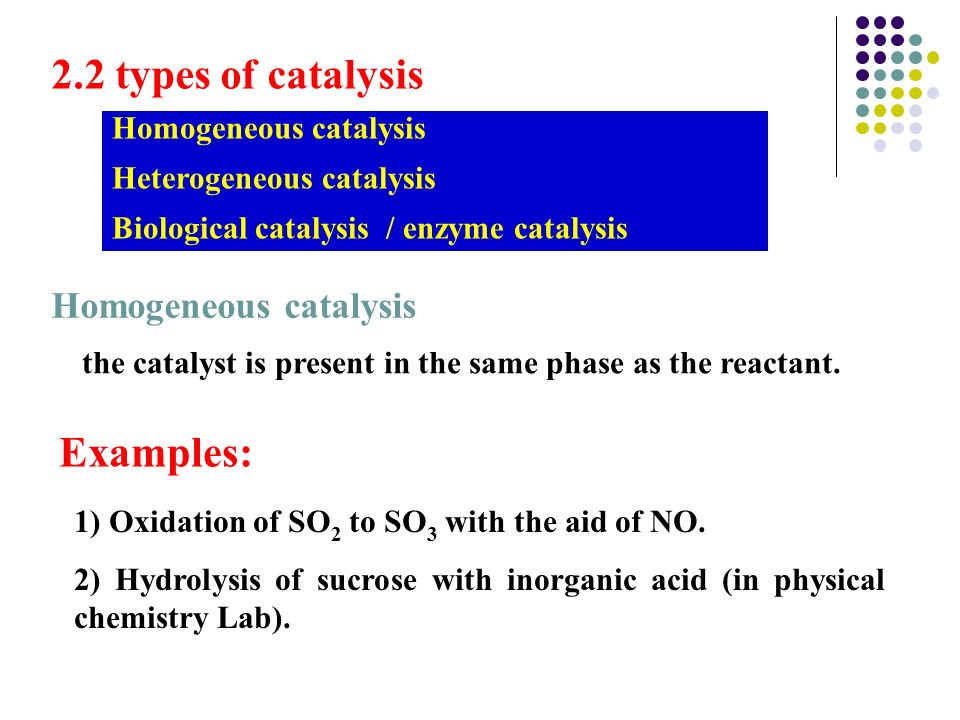
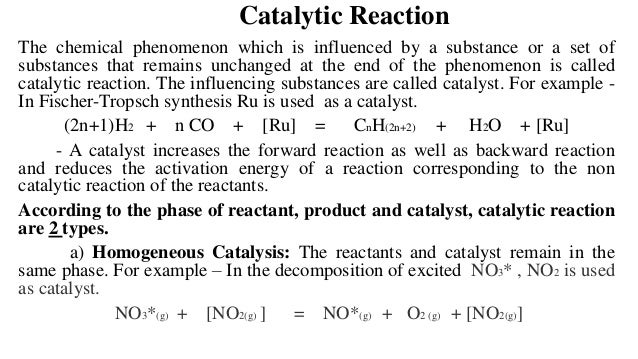
For example hydrolysis of sugar in the presence of sulphuric acid Heterogeneous Catalysis Heterogeneous catalysis of chemical reactions is a process where the reactants involved in the reaction and the catalyst are in different phases For example reaction of hydrogen and nitrogen in the presence of finely divided iron to form ammoniaHeterogeneous catalysis has traditionally been used for the largescale production of relatively simple molecules—for example, the Haber Bosch process to convert nitrogen and hydrogen into ammonia using Febased catalysts or catalytic cracking of long chain hydrocarbons using zeolites2H2O2 (l) →Pt(s) 2H2O (l) O2(g)In this reaction, reactant and catalyst are in different phase, hence it is an example of heterogeneous catalysis Heterogeneous catalysis is a type of catalysis in which the catalyst occupies a different phase from the reactants and products Itmay refer to the physical phase solid, liquid or gas but also to immiscible fluids
Catalysis Ppt Video Online Download
What is heterogeneous catalysis give an example
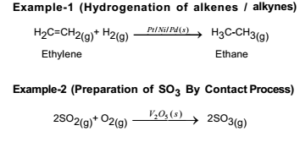
What is heterogeneous catalysis give an example-Examples of heterogeneous catalysis The hydrogenation of a carboncarbon double bond The simplest example of this is the reaction between ethene and hydrogen in the presence of a nickel catalyst In practice, this is a pointless reaction, because you are converting the extremely useful ethene into the relatively useless ethane However, the same reaction will happen with any144 million tons of ammonia were produced in 16
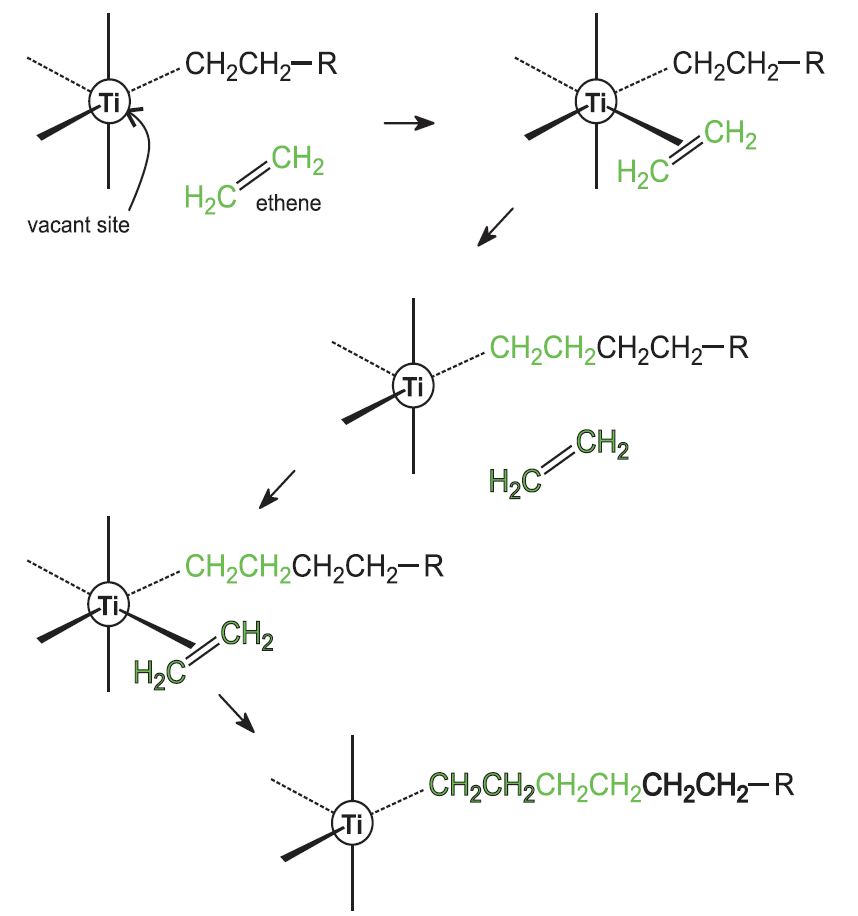



Catalysis In Industry
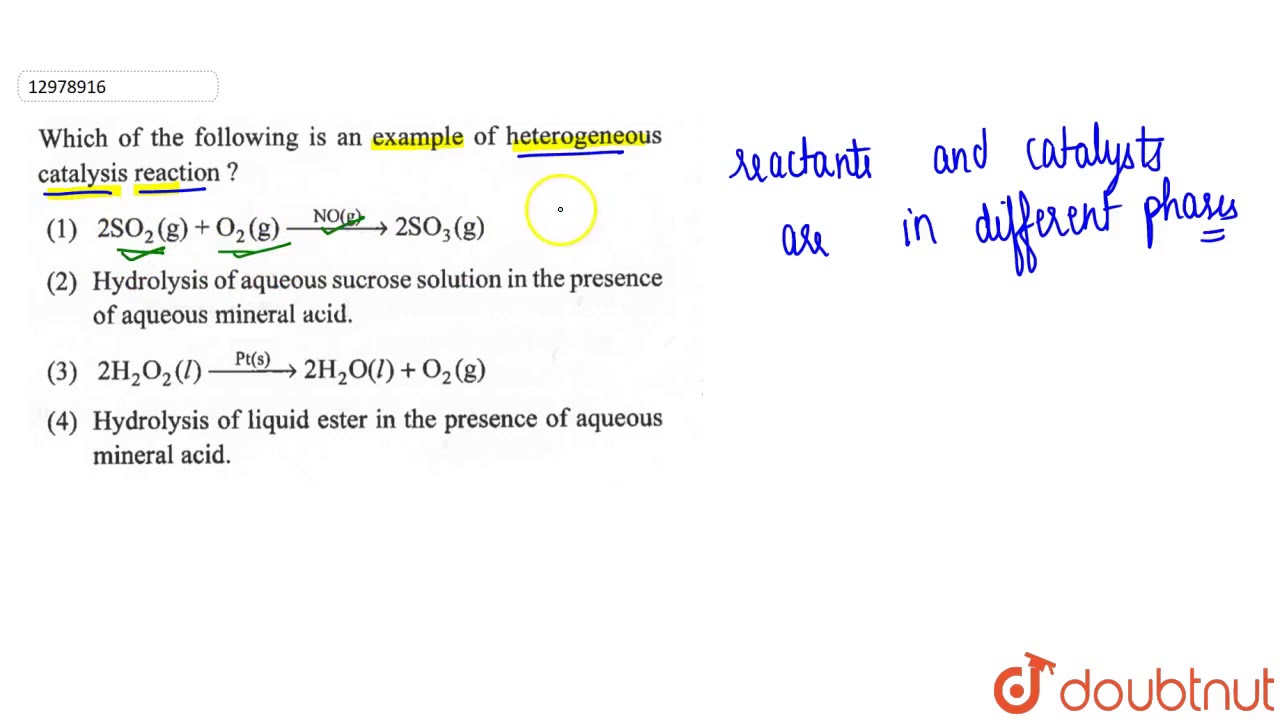
In which the reactants and catalyst are in a similar phase Example 2SO 2 (g) O 2 (g) —– No(g) → 2SO 3 (g) Heterogeneous catalysis Heterogeneous catalysts are catalytic compounds that are in a contradictory phase from that of the phase of the reaction combination Heterogeneous catalysis is found in the liquid phase, gas phase, and solid phaseWhat is Heterogeneous Catalysis and Catalysts?The catalyst whose phase differs from that of the reactants in the reaction is called heterogeneous catalyst and this type of catalysis process is called heterogeneous catalysis Examples of Heterogeneous Catalysis and Catalysts – 1 In Haber's process of formation of ammonia, nitrogen and hydrogen are used in gaseous forms
As an example of a detailed mechanism for heterogeneous catalysis at a surface, when oxygen and hydrogen combine on the surface of titanium dioxide (TiO 2, or titania) to produce water Scanning tunneling microscopy showed that the molecules undergo adsorption, dissociation, and diffusion before reacting12 K views 800 people like this Like Share Share Answer Text `2SO_2(g)O_2(g)overset (NO(g)) to 2SO_2(g)`Hydrogen of aqueousWatch Video in App This browser does not support the video element 663 k 560 k Answer Step by step solution by experts to help you in doubt clearance & scoring
Heterogeneous catalysis constituted one of the earliest purposed applications for crystalline MetalOrganic Frameworks (MOFs) 1 The MOF concept was introduced in the literature by Yaghi's research group 2, being these materials, in the most elementary sense, obtained by connecting together metal ions with organic linkers, often resulting in fascinating structuralThe chemical and energy industries rely heavily on heterogeneous catalysis For example, the Haber–Bosch process uses metalbased catalysts in the synthesis of ammonia, an important component in fertilizer;UNESCO – EOLSS SAMPLE CHAPTERS INORGANIC AND BIOINORGANIC CHEMISTRY – Vol II Homogeneous and Heterogeneous Catalysis Erica Farnetti, Roberta Di Monte and Jan Kašpar ©Encyclopedia of Life Support Systems (EOLSS) terms ab,, and cd,, represent the stoichiometric coefficients of the reactionFor such a reaction we can define the reaction rate as



Perspective On Computational Reaction Prediction Using Machine Learning Methods In Heterogeneous Catalysis Physical Chemistry Chemical Physics Rsc Publishing




Catalysis In Industry
Reaction (I) and (III) are heterogeneous catalysis since reacting species and products are in the gas phase and catalyst is in the solid phase So, the correct option is A Video ExplanationHeterogeneous acid catalysts are synthesized as insoluble salts or solid matrices of high surface area containing an active dopant, in most times via solgel processes, precipitation or impregnation methods Currently, biodiesel production processes employing heterogeneous catalysis require more severe working conditions than those used in the homogeneous ones (ie, higherHomogeneous and heterogeneous catalysis Activity is the ability of the catalyst to accelerate a chemical reaction The degree can be as high as 100 times in certain reactions A catalytic cycle processes in which the reactant and catalyst undergo several transformations before making the
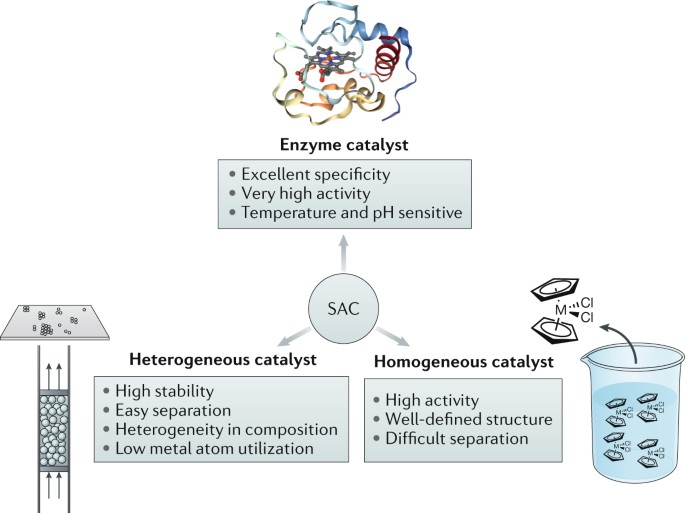



Heterogeneous Single Atom Catalysis Nature Reviews Chemistry



Catalysis In Industry
16 Heterogeneous Catalysis Systems and Surface Reactions¶ RMG can now be used to study heterogenous catalysis and surface reactions Initially developed in a fork of RMG called RMGCat (Goldsmith17), this is now a part of the main RMG softwareSeveral surface specific features need to be considered when setting up an input file for surface reaction mechanism generation, An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as Ni, Pd, or Pt Because the relatively strong H–H bond (dissociation energy = 432 kJ/mol) has already been broken, the energy barrier for most reactions of H 2 is substantially lower on the catalyst surfaceIt is said to be heterogeneous and the catalysis is hetergenous catalyst Example of hetergeneous Catalyst is manufacture of in contact process using as catalyst Usally in a hetergenous catalyst the reactant are gases and reaction starts from the surface of the solid catalyst this is the reason why hetergeneous catalyst is also called



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora




Solid Phase Catalysis In Continuous Flow Syrris Chemistry Blog
Most of the metals used in heterogeneous catalysis are organised in a facecentred cubic (fcc) tightly packed crystal structure in which each atom has a maximum of 12 nearest neighbours The metal atoms at the surface are the only ones presenting vacancies where the reactants can bind For this reason a catalyst made in such a way that the metal particles are highly dispersed on aUpdated On 136 To keep watching this video solution for FREE, Download our App Join the 2 Crores Student community now!Catalysis' research aims at investigating catalytic activity of new homogeneous organometal complexes and other heterogeneous or homogeneous catalytic materials europaeu les re ch erches menées dans le do ma ine de l a catalyse o nt p our ob jet l'étude de l'activité catalytique de nouveaux complexes organométalliques et d'autres matériaux c atal ytiq ue s hétérogènes o u



1 An Introduction To Types Of Catalysis Chemistry Libretexts




Heterogeneous Catalysis Wikipedia
PDF Was reviewed acid, basic and metalic catalyst sites activity in heterogeneous catalysis Find, read and cite all the research you need on ResearchGateHomogeneous and heterogeneous catalysis differ inphase in which the starting materials are located If the initial components taken for interactions, including the catalyst, are in the same aggregate state, homogeneous catalysis proceeds In the case when different phase substances participate in the reaction, heterogeneous catalysis takes place Selectivity of action CatalysisAnswer The catalytic process in which the reactants and the catalyst are in different phases is known as heterogeneous catalysis examples of heterogeneous catalysis are given belowOxidation of sulphur dioxide into sulphur trioxide in thepresence of Pt2SO2 (g) →Pt(s) 2SO3 (g) The reactant is in gaseous state while the catalyst is in the solid state




Difference Between Catalytic And Non Catalytic Reaction Compare The Difference Between Similar Terms



Question 8 10 Points A What Is The Difference Chegg Com
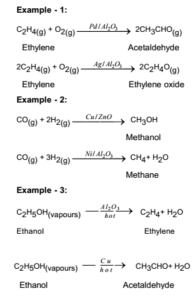
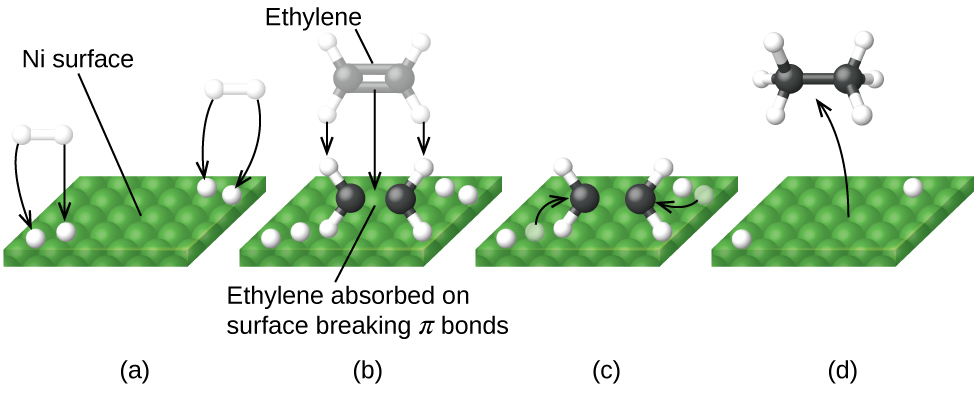
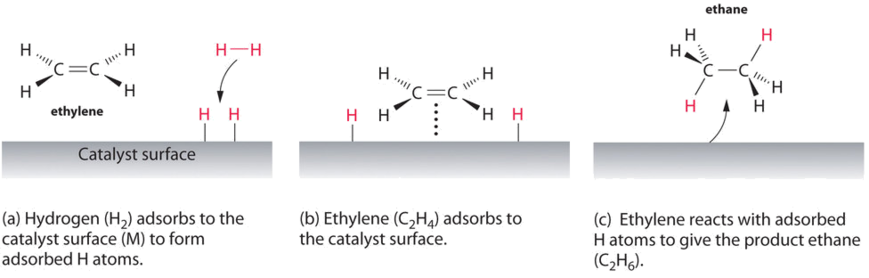
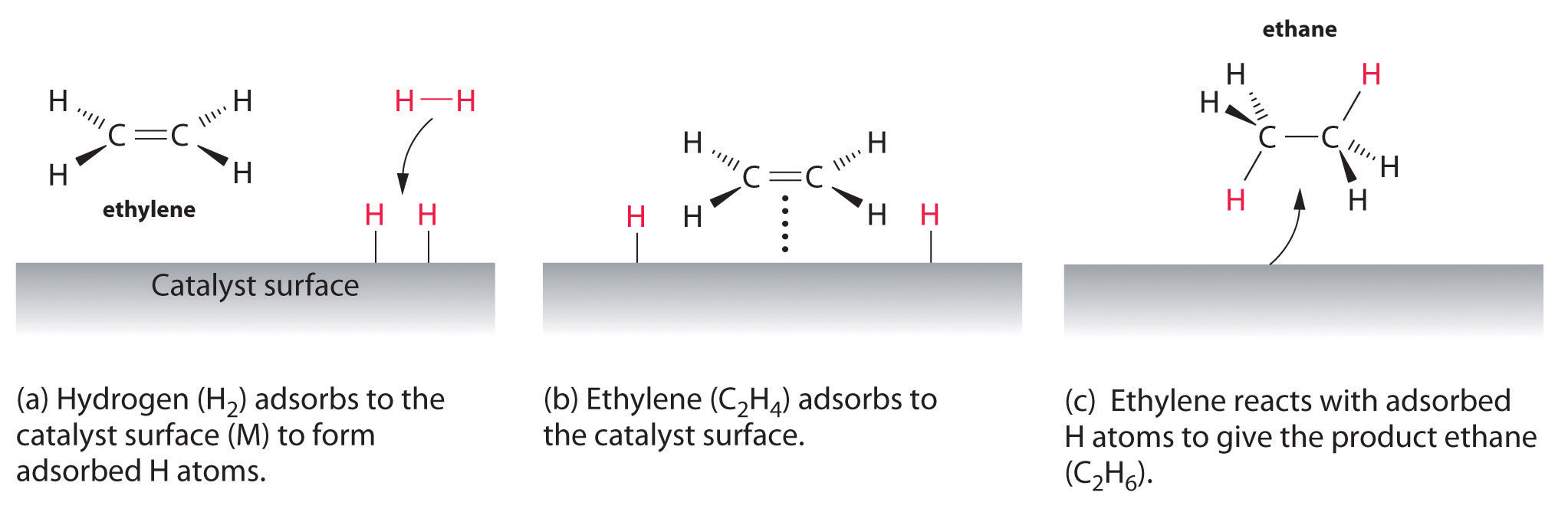
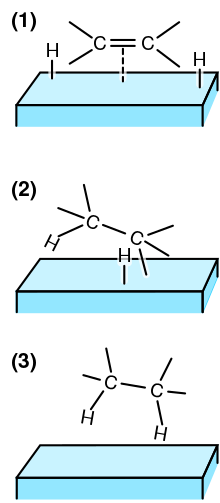
In heterogeneous catalysis, chemisorption is usually a necessary part of the catalytic reaction Therefore, this is the only type of adsorption that we discuss here The most common description of chemisorption in heterogeneous catalysis is through the Langmuir isotherm The theory for the Langmuir isotherm is based on the following assumptions In heterogeneous catalysis, catalyst is generally a solid and the reactants are generally gases This is also known as surface catalysis because the reaction starts at the surface of the solid catalyst These catalysts have very large surface area of the order of 1 to 500 m2 per gram for contact Thus, despite an enormous surface area, once the reactant gas molecules One example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (C 2 H 4) by metal catalysts such as Pt or Ni The currently accepted mechanism for this reaction involves weak bonding of both H 2 and C 2 H 4 to atoms on the metal surface This is called adsorption




Heterogeneous Catalysis Wikipedia




Catalysis Ppt Video Online Download
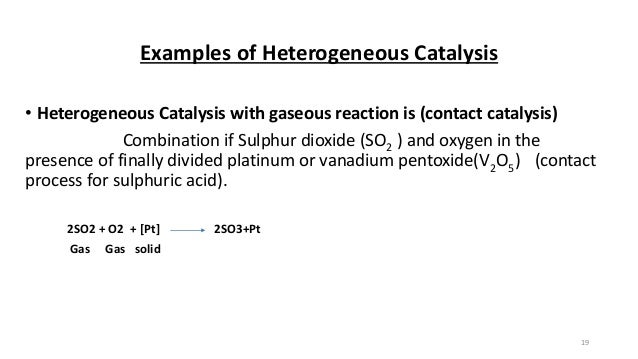
Heterogeneous catalysis is the type of catalysis where the phase of the catalyst differs from the phase of the reactants or products Contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase The production of 90% of chemicals (by volume) is assisted by solid catalysts Similarly, what does a phase transfer catalyst do?Get answer Which one of the following reaction is an example of heterogeneous catalysis?Some common examples of reactions that involve heterogeneous catalysis (reactions in which the physical states of the reactants and the catalysts are different) are provided below The contact process for the synthesis of sulfuric acid, which involves the reaction between oxygen and sulfur dioxide, catalyzed by oxides of vanadium




26 Homogeneous And Heterogeneous Catalysis Chapter




Types Of Catalysts Article Kinetics Khan Academy
Heterogeneous Catalytic Reaction English An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal such as Ni Pd or Pt The metal probably increases reaction rate by either holding reactant molecules in the correct orientation to react or by weakening or breaking bonds in reactant molecules to make them more reactive This is an example of heterogeneous catalysis It is heterogeneous catalysis because the catalyst is a solid and the reactants are gases Examples Then the mixture of the liquid substrate and solid catalyst is shaken or stirred in a hydrogen atmosphere However, then actual reaction takes place at the surface of the metal catalyst and is an example of heterogeneous or surface catalysis



Catalysis Meaning Of Catalyst Its Characteristics And Types



12 7 Catalysis Chemistry
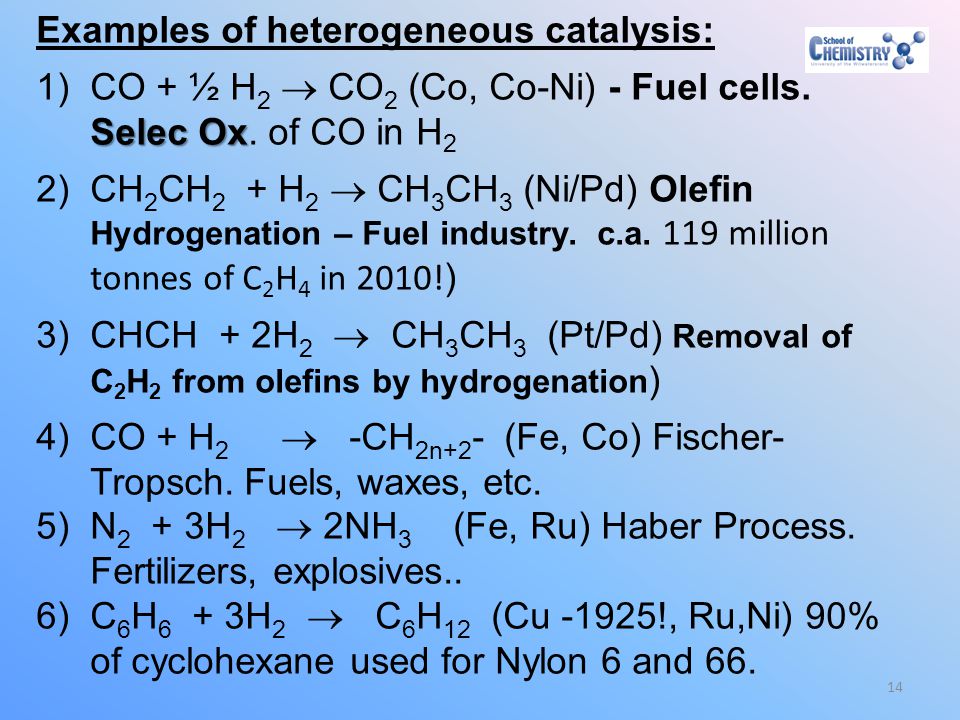
Give four examples of heterogeneous catalysis Answer (i) Oxidation of sulphur dioxide to form sulphur trioxide In this reaction, Pt acts as a catalyst (ii) Formation of ammonia by the combination of dinitrogen and dihydrogen in the presence of finely divided iron This process is called the Haber's process (iii) Oswald's process Oxidation of ammonia to nitric oxide in theWhich of the following is an example of heterogeneous catalysis reaction ?Finely divided metals, metal gauzes, metals incorporated into supporting matrices, and metallic films have all been used in modern heterogeneous catalysis




What Are Some Examples Of Homogeneous Catalysis Quora




Complexities In Modeling Of Heterogeneous Catalytic Reactions Sciencedirect
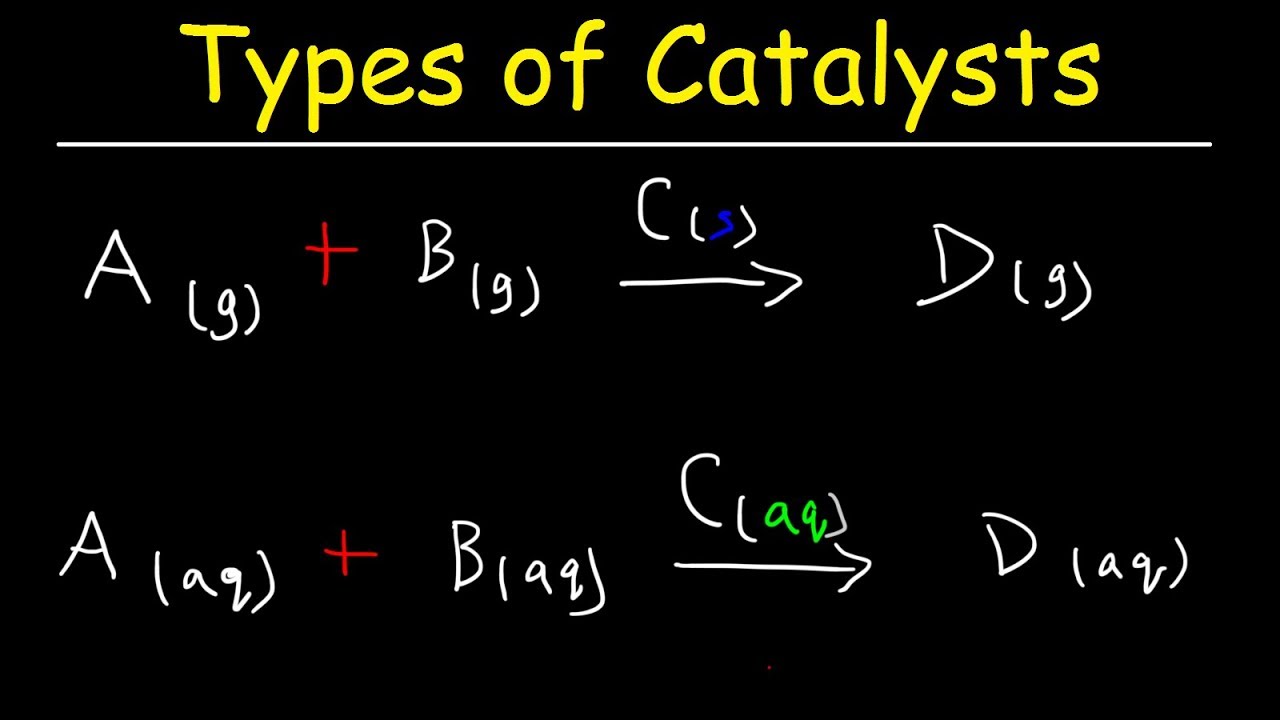
Homogeneous vs heterogeneous catalysis Dr habil Marko Hapke 3 3 Heterogeneous Catalysis Homogeneous Catalysis Catalyst and reactant(s) are in the same phase Catalyst and reactant(s) are in different phases Definitions General features Different reaction phases possible „classic"Get answer What is heterogeneous catalysis ?Difference Between Homogeneous Catalysis and Heterogeneous Catalysis Video Lecture from Surface Chemistry Chapter of Chemistry Class 11 for HSC, IIT JEE, CBS




Adsorption Theory Of Heterogeneous Catalyst Definition Examples



14 7 Catalysis Chemistry Libretexts
Future Challenges in Heterogeneous Catalysis Understanding Catalysts under Dynamic Reaction Conditions Kai F Kalz,a Ralph Kraehnert,b Muslim Dvoyashkin,c RolandDittmeyer,d Roger Glser,c UlrikeKrewer,e Karsten Reuter,f and JanDierk Grunwaldt*a, g 1 Introduction At the sight of depleting fossil fuel reserves and facing increasing environmental problems connectedDehydrogenation by Heterogeneous Catalysts Daniel E Resasco School of Chemical Engineering and Materials Science University of Oklahoma Encyclopedia of Catalysis January, 00 1 1 INTRODUCTION Catalytic dehydrogenation of alkanes is an endothermic reaction, which occurs with an increase in the number of moles and can be represented by the expression Alkane !Give an example Solution At catalytic process in which the reactants and the catalyst are in different phases is known as heterogeneous catalysis Example (1) Oxidation of sulphur dioxide to sulphur trioxide in presence in



Heterogeneous Catalysis And Catalyst Recycling All About Drugs



Heterogeneous Catalysis All About Drugs
Olefin Hydrogen This reactionIn homogeneous catalysis, products cannot be separated while in heterogeneous catalysis the final product can be separated Hydrogen gas is evolved in the reaction between zinc and dilute sulfuric acid NCERT P Bahadur IITJEE Previous Year Narendra Awasthi MS Chauhan In a reaction, if the catalyst is present in the same phase as the reactants, it is called a homogeneous catalyst This example brings out a number of facets of heterogeneous catalysis The existence of a macs, which suppresses all but one of the Langmuir denominator terms, is the simplest possible explanation for an observed rate that fits a power law The macs can be the unoccupied surface However, unless the surface reaction is singlestep, a mucs does not




Heterogeneous Catalyst An Overview Sciencedirect Topics



Q Tbn And9gctrvbbisy7ikjyh35 8msk3jkudkzizuszlwrim5fn3f2i54ygk Usqp Cau
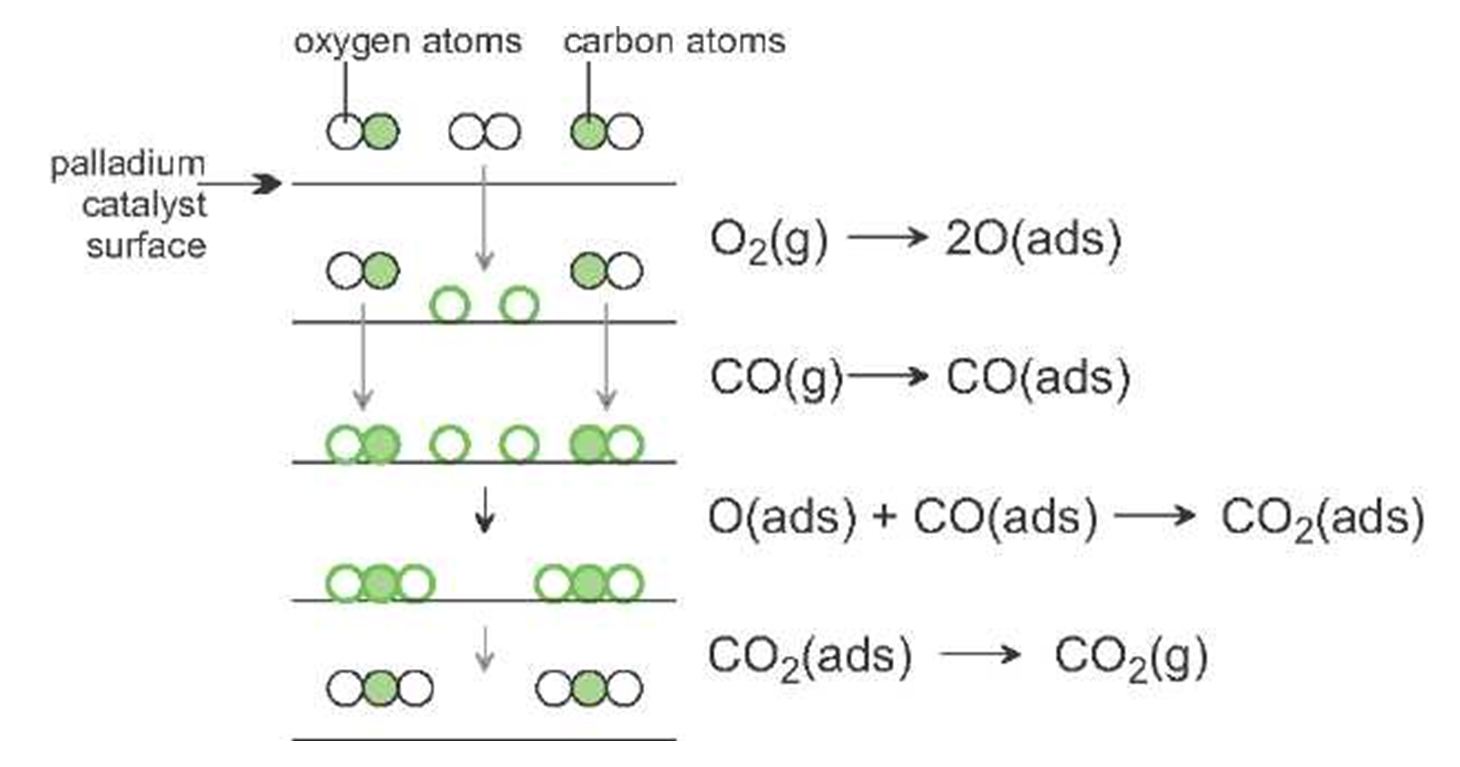
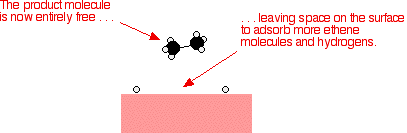
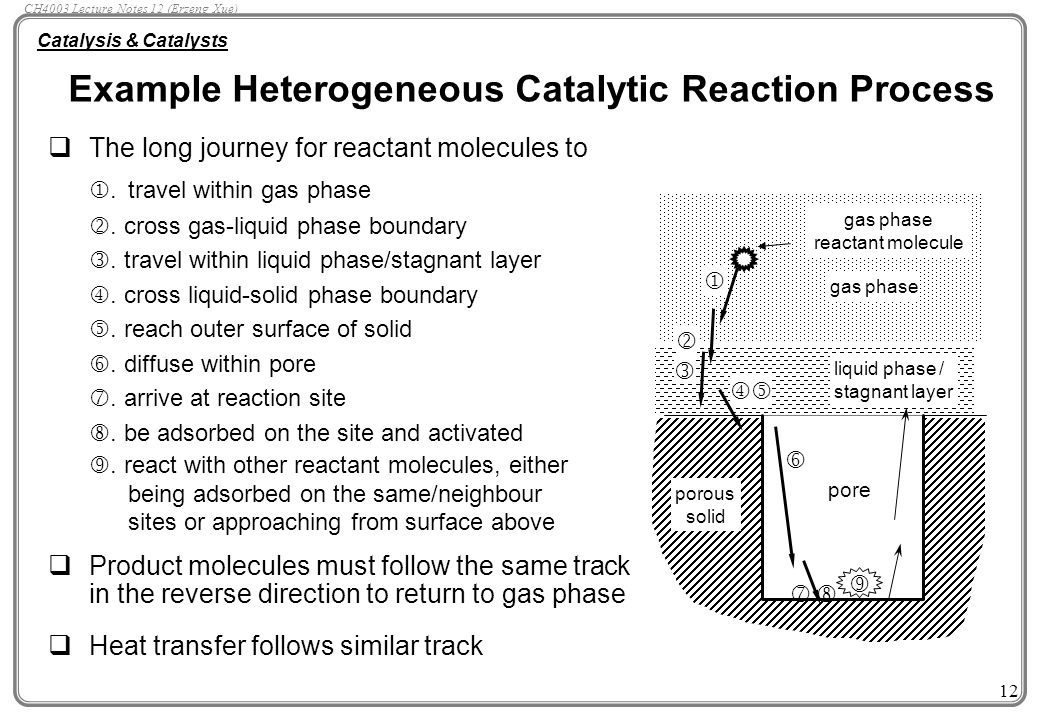
12 Mechanism of Chemical Reactions on Solid Catalysts Step 1 Transport of reactants from the bulk fluid to the fluidsolid interface Step 2 Intraparticle transport of reactants into the catalyst particle ( If it is porous ) Step 3 Adsorption of reactants at interior of the catalyst particleAn example of the stepwise processes that occur in heterogeneous catalysis is the oxidation of carbon monoxide to carbon dioxide over palladium This is a very important process in everyday life Motor vehicles are fitted with catalytic converters These consist of a metal casing in which there are two metals, palladium and rhodium, dispersed very finely on the surface of a ceramic support In heterogeneous catalysis, the reaction starts at the surface of the solid catalyst and so it is also known as surface catalysis Example Manufacture of H 2 SO 4 by contact process involves oxidation of SO 2 into SO 3 in presence of V 2 O 5 (solid) as catalyst



Catalysts Free Full Text Heterogeneous Catalyst Deactivation And Regeneration A Review Html



Types Of Catalysis
Which of the following is an example for heterogeneous catalysis reaction?Apne doubts clear karein ab Whatsapp par bhi Try it now CLICK HERE 1x 15x 2x Loading DoubtNut Solution for you Watch 1000 concepts & tricky questions explained!These are known as heterogeneous catalytic reactions They include reactions between gases or liquids or both at the surface of a solid catalyst Since the surface is the place at which the reaction occurs, it generally is prepared in ways that produce large surface areas per unit of catalyst;



1
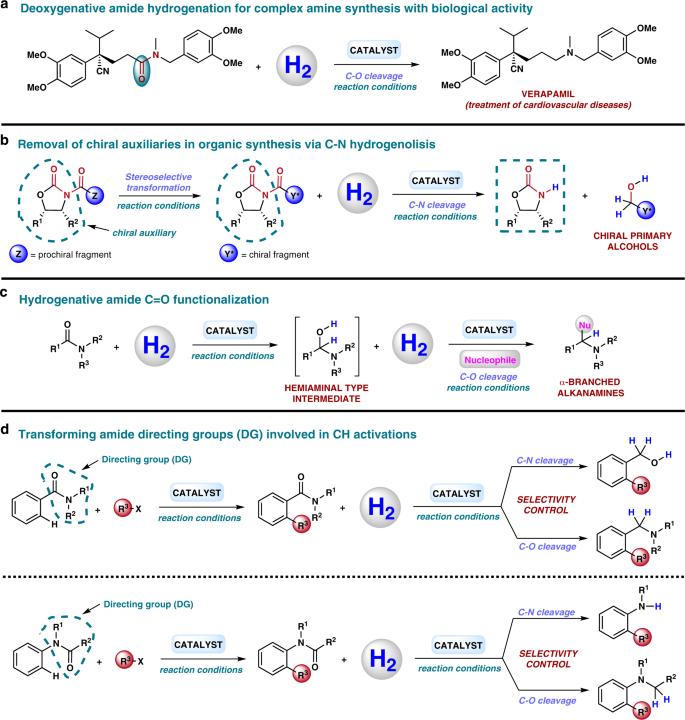



Homogeneous And Heterogeneous Catalytic Reduction Of Amides And Related Compounds Using Molecular Hydrogen Nature Communications
Click here👆to get an answer to your question ️ Which of the following is an example for heterogeneous catalysis reaction?
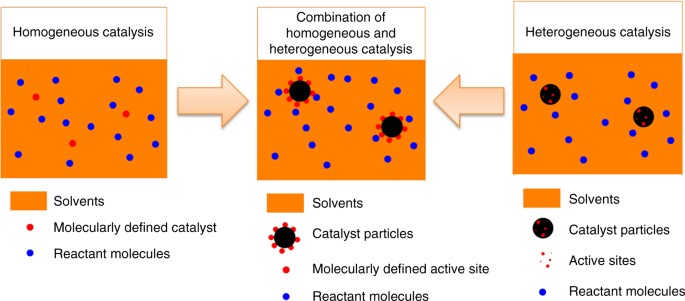



Synthesis Of A Molecularly Defined Single Active Site Heterogeneous Catalyst For Selective Oxidation Of N Heterocycles Nature Communications
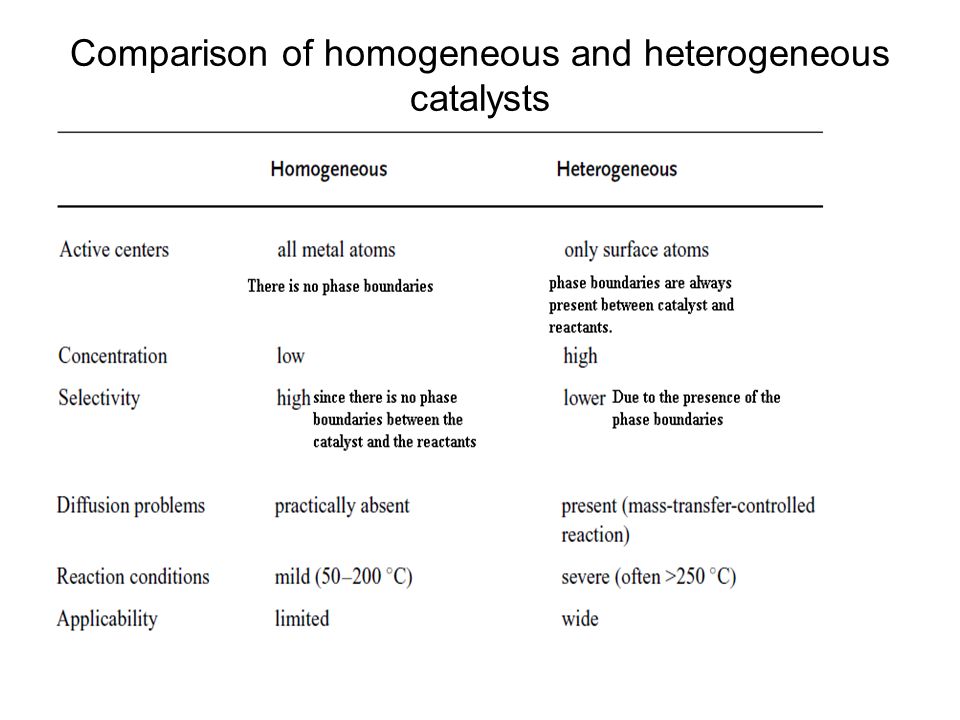



Heterogeneous Catalysis Ppt Video Online Download




Heterogeneous Catalysis An Overview Sciencedirect Topics




Which Of The Following Is An Example For Heterogeneous Catalysis Reaction
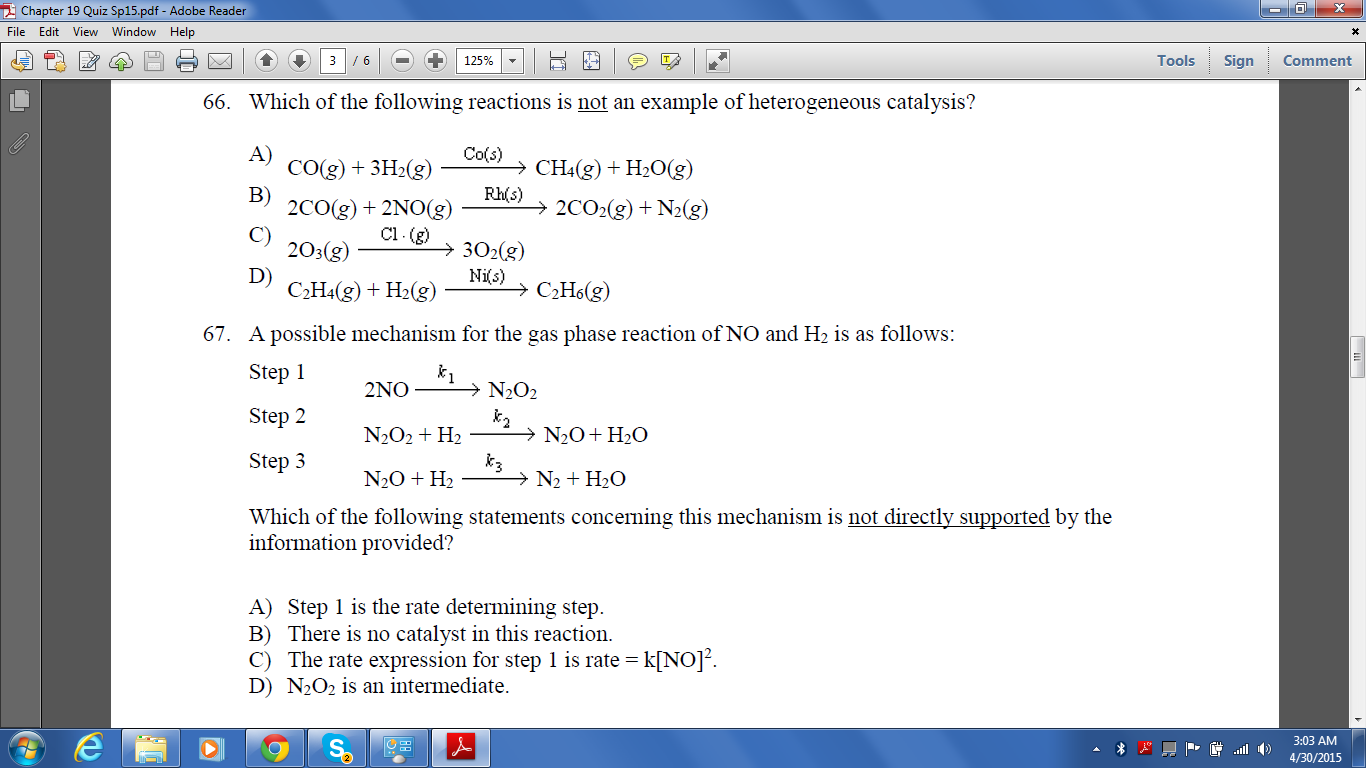



Chapter 19 Quiz Adobe Read File Edit View Window Help Chegg Com
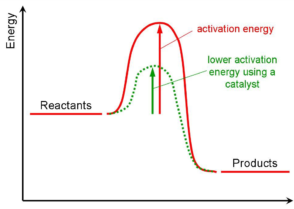



Catalysis Meaning Of Catalyst Its Characteristics And Types



Types Of Catalysis




Chapter 13 8 Catalysis Chemistry Libretexts




Homogeneous Catalysis Introduction To Chemistry



The Catalytic Action Of Nitric Oxide To Break Down Chegg Com



Http Www Umich Edu Elements 6e Powerpoints 13lectures Lec27 Pdf Pdf



Which Of The Following Is An Example Of Heterogeneous Catalysis Reaction Youtube



Homogeneous Vs Heterogeneous Catalysts Basic Introduction Youtube



Catalysis Catalysts Facts And Figures About Catalysts Ppt Download




Homogeneous Catalysis Wikipedia




Catalysis Boundless Chemistry




Heterogeneous Catalysis Sciencedirect




Which Of The Following Reactions Is Not An Example Of Chegg Com




Heterogeneous Metal Catalysts For Oxidation Reactions




Heterogeneous Metal Catalysts For Oxidation Reactions



Catalysis



Promoting Heterogeneous Catalysis Beyond Catalyst Design Chemical Science Rsc Publishing




Catalysis Wikipedia



10 5 Catalytic Reaction Ppt Video Online Download



Heterogeneous Catalysis All About Drugs



Shoshi Catalytic Non Catalytic Reactions



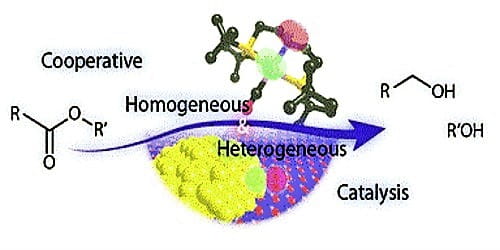
Combining Homogeneous And Heterogeneous Catalysis Feature Chemistry World
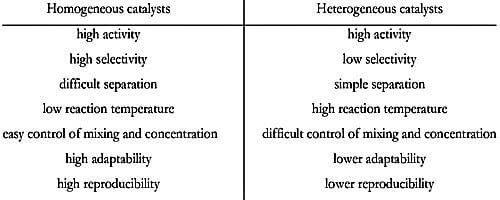



Differences Between Homogeneous Catalysis And Heterogeneous Catalysis Qs Study




Give Four Examples Of Heterogeneous Catalytic Reactions Youtube
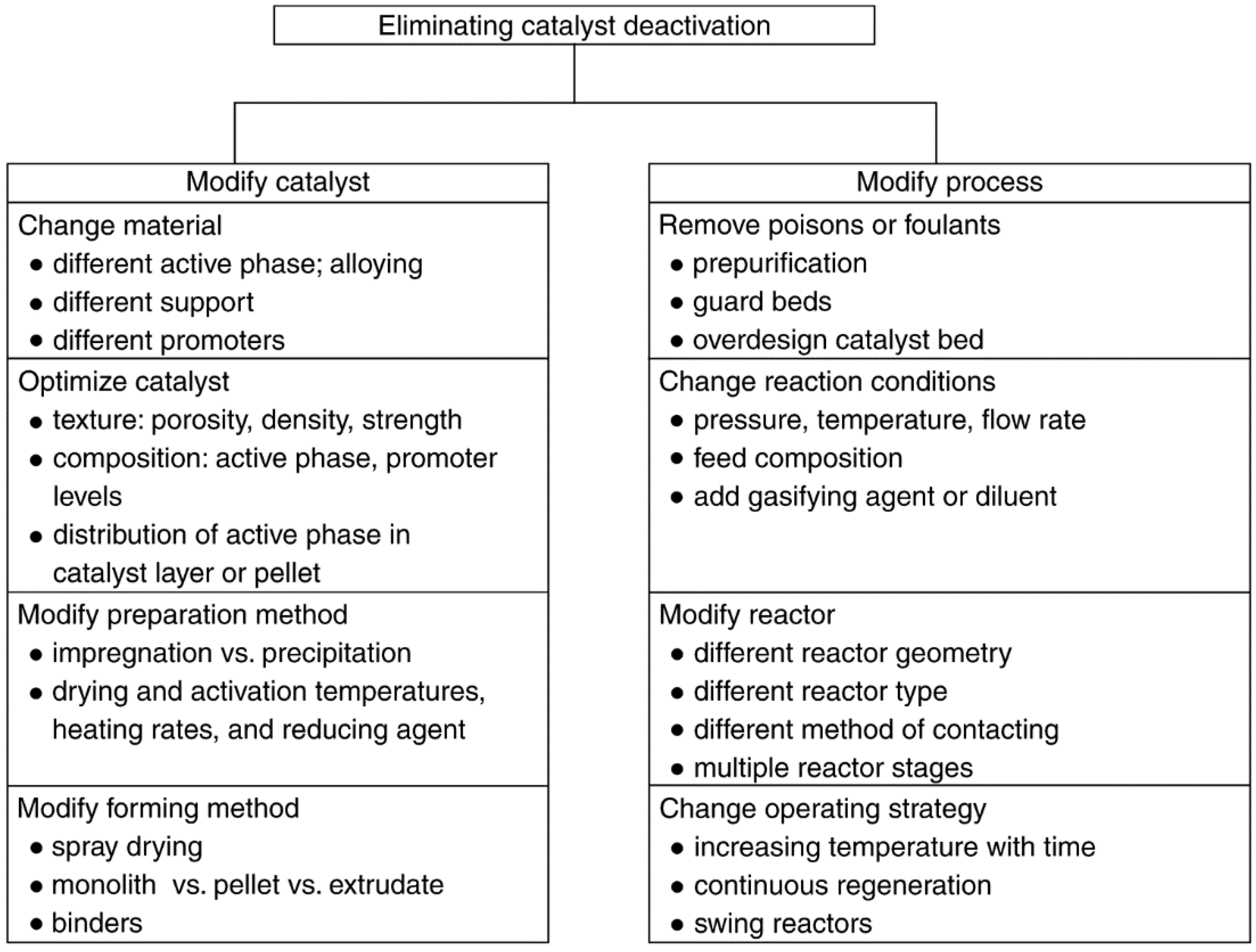



Catalysts Free Full Text Heterogeneous Catalyst Deactivation And Regeneration A Review Html




Which Of The Following Is An Example Of Heterogeneous Chegg Com



Catalysis
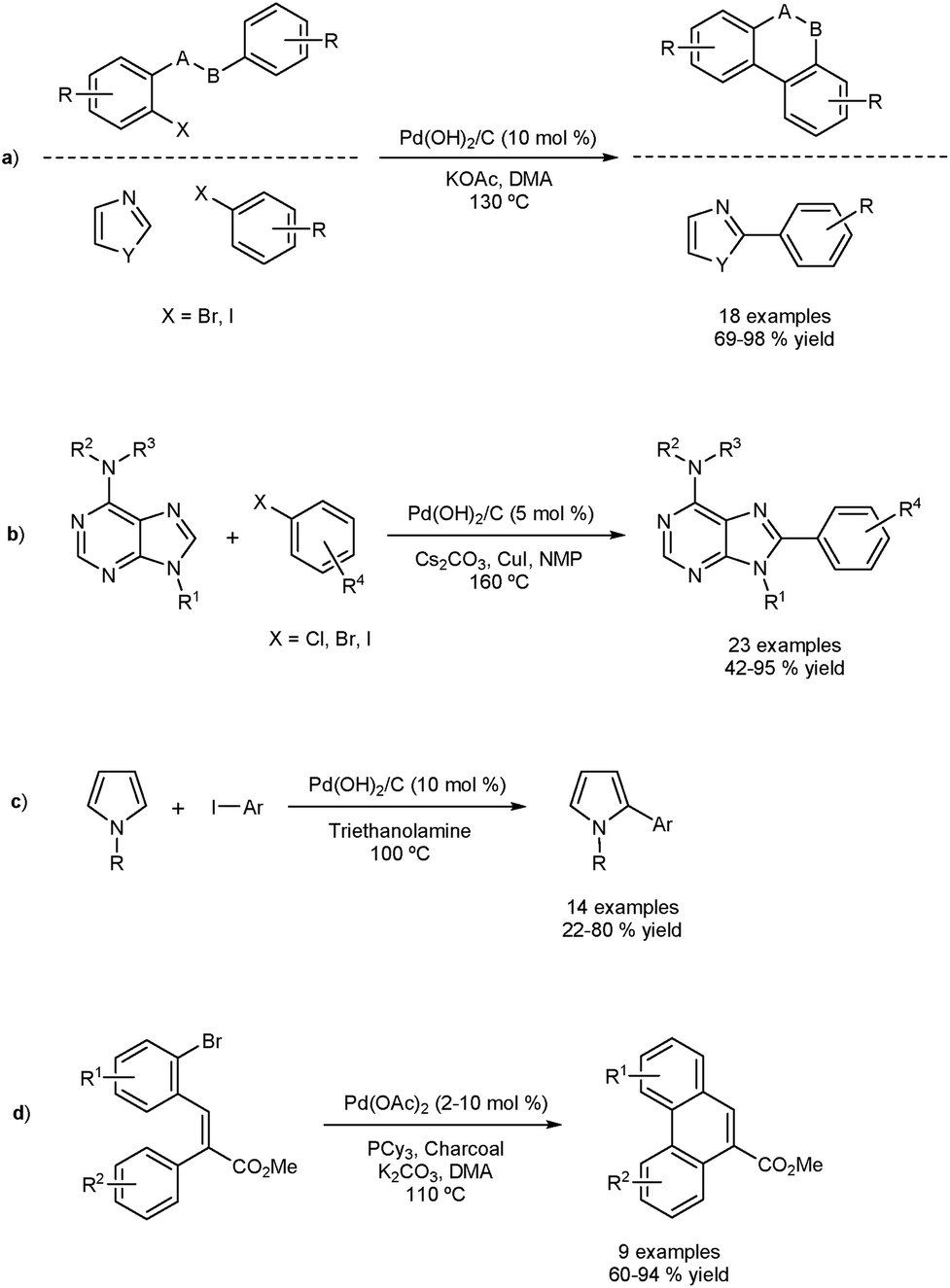



Direct Arylation And Heterogeneous Catalysis Ever The Twain Shall Meet Chemical Science Rsc Publishing




Homogeneous Catalysis Catalysis Heterogeneous Catalysis




Catalysis Fundamentals Chemical Engineering Page 1




Kinetics Theory Of Catalytic Mechanisms Heterogeneous Catalysis Homogeneous Catalyzed Reaction Examples Advanced A Level Gce Revision Notes
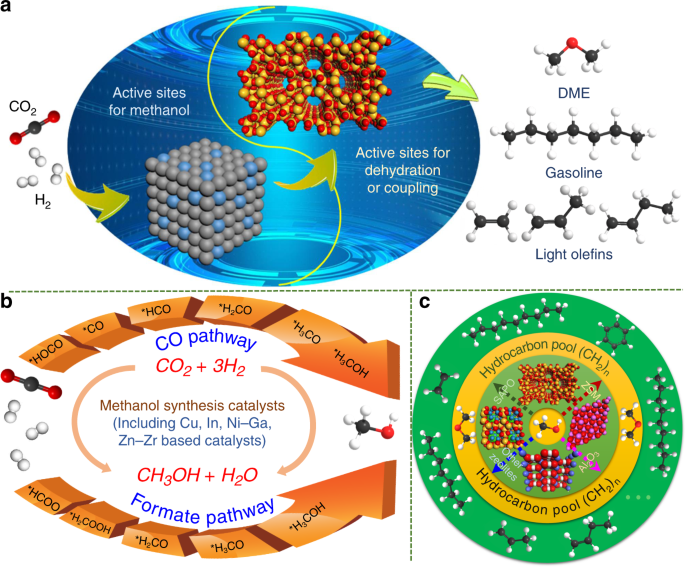



Co2 Hydrogenation To High Value Products Via Heterogeneous Catalysis Nature Communications




Individual Steps Of A Simple Heterogeneous Catalytic Fluid Solid Download Scientific Diagram



Chemistry 3030 Catalysis Course Ppt Download
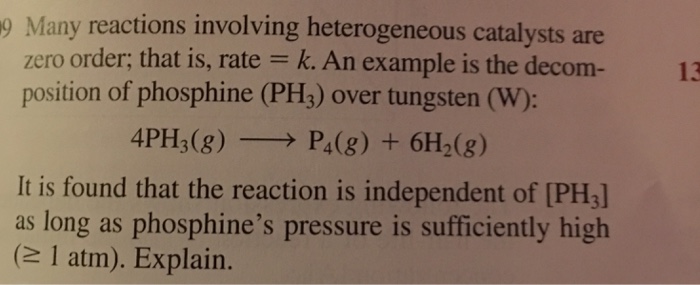



Many Reactions Involving Heterogeneous Catalysts Are Chegg Com



1




Catalyst Ppt Video Online Download




Heterogeneous Metal Catalysts For Oxidation Reactions




What Is Heterogeneous Catalysis Give An Example
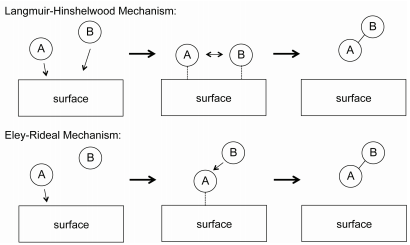



Lecture 23 Kinetics Of Catalysis Chemistry Libretexts
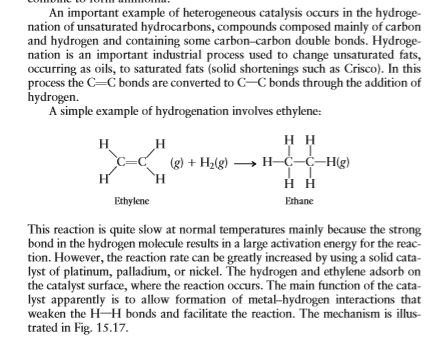



An Important Example Of Heterogeneous Catalysis Chegg Com




Crossing The Divide Between Homogeneous And Heterogeneous Catalysis In Water Oxidation Pnas



Principles Of Heterogeneous Catalysis Dumesic Major Reference Works Wiley Online Library
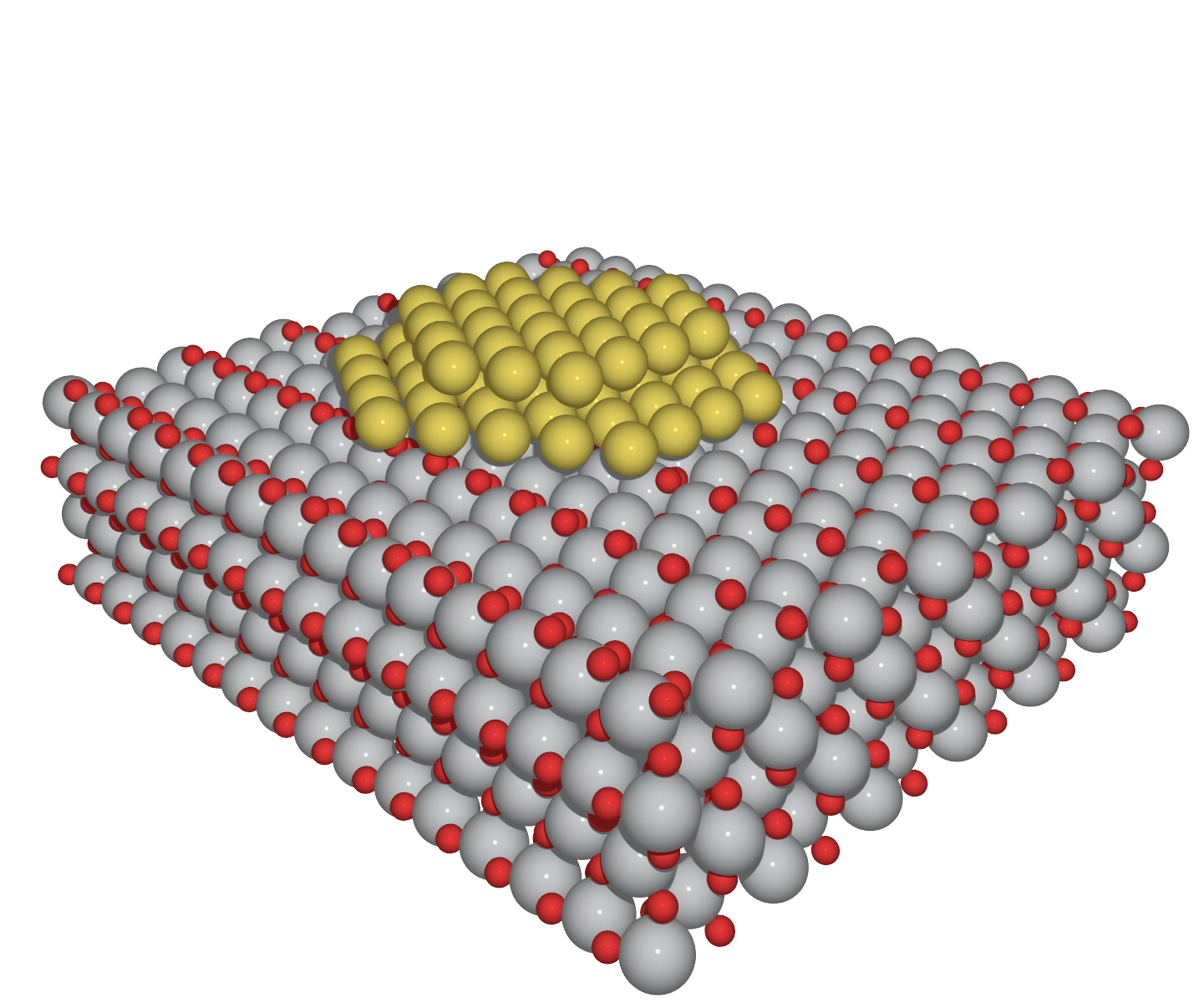



Heterogeneous Gold Catalysis Wikipedia




Pdf Heterogeneous Catalytic Chemistry By Example Of Industrial Applications Semantic Scholar




Heterogeneous Homogeneous Catalysts Video Lesson Transcript Study Com




Catalysis Powerpoint Slides



Catalysis Meaning Of Catalyst Its Characteristics And Types




Heterogeneous Catalysis Wikipedia



Heterogeneous Catalysis Qs Study




Difference Between Homogeneous Catalysis And Heterogeneous Catalysis Surface Chemistry Youtube




Which Of The Following Reations Are Examples For Heterogeneous Catalysis Youtube



Heterogeneous Catalysts A Brief Recount Of The Reasons And The Justification That S Support Theoretical Simulations By Jesus M Garcia Figueroa Uprm Department Of Chemical Engineering



Heterogeneous Catalysis Wikipedia



Differences Between Homogeneous Catalysis And Heterogeneous Catalysis Qs Study




Which Of The Following Reactions Is An Examples Of Homogeneous Catalysis Youtube
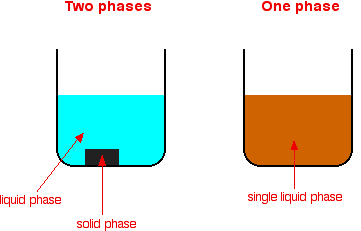



Types Of Catalysis




Give Four Examples Of Heterogeneous Catalysis



1



Types Of Catalysis Homogeneous Catalysis Heterogeneous Catalysis Positive Catalysis Negative Catalysis Induced Catalysis Acid Base Catalysis




Homogeneous Catalysis An Overview Sciencedirect Topics




Pdf Heterogeneous Catalytic Chemistry By Example Of Industrial Applications Semantic Scholar




Solid Phase Catalysis In Continuous Flow Syrris Chemistry Blog
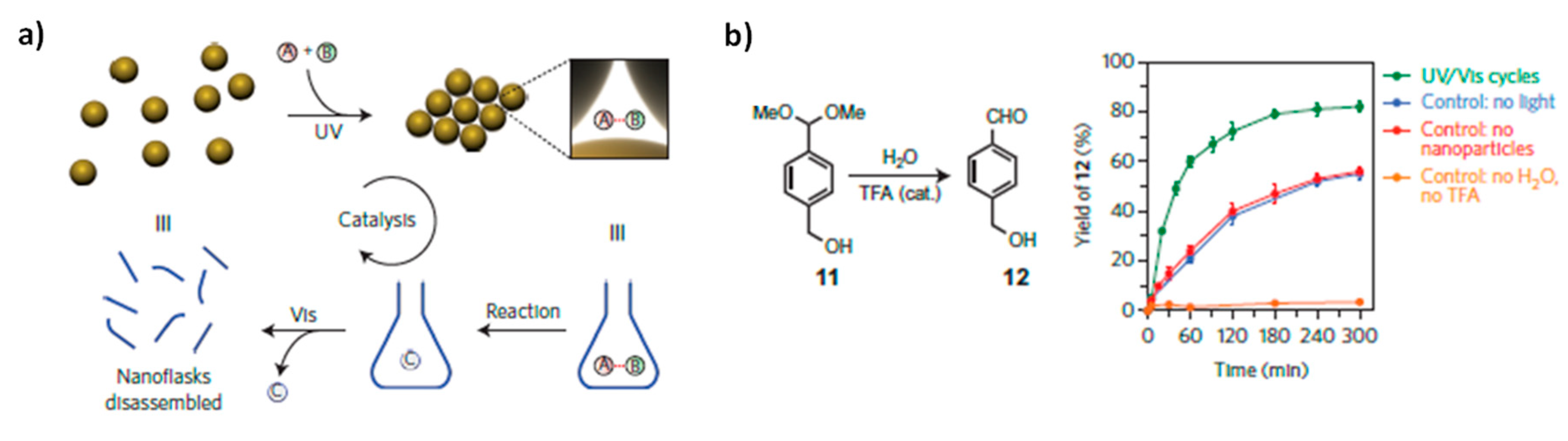



Catalysts Free Full Text Switchable Stimuli Responsive Heterogeneous Catalysis Html




Evolution Of Isolated Atoms And Clusters In Catalysis Trends In Chemistry
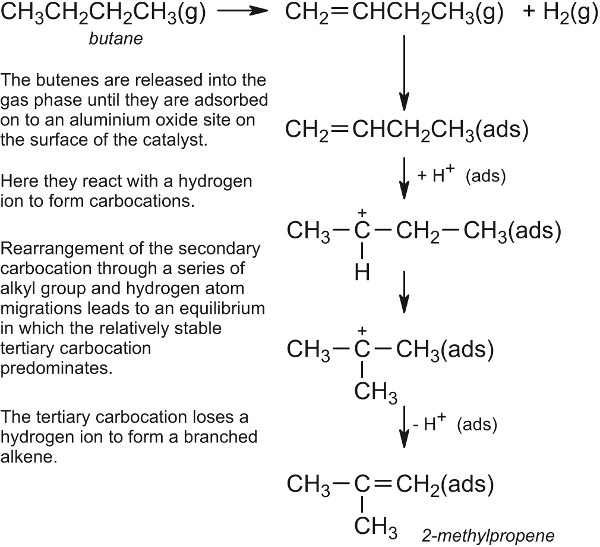



Catalysis In Industry




Catalysis Heterogeneous Catalysis Britannica




Which Of The Following Is Not An Example Of Heterogeneous Catalytic Reaction Youtube




Heterogeneous Catalysis Alchetron The Free Social Encyclopedia




Examples Of Tin Based Heterogeneous Catalysts Used In The Download Table




Heterogeneous Catalysis Wikipedia


